Technology Development Milestones
A timeline of our key milestones and technological breakthroughs.
1993
Start Hepatocyte Culture
%EB%B0%B0%EC%96%91.png&w=828&q=75)
1999
Expand into Organotypic Liver Culture
.png&w=828&q=75)
2003
Design Provisional Matrix-Mimicking Hydrogel
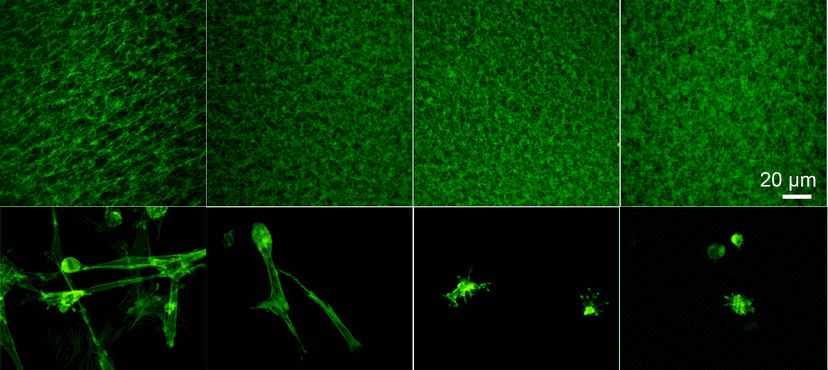
2004
Discover Adipose Stem Cells-Integrated Biofiller & Optimize a Chemical-Define Cryopreservation recipe
2006
Succeed to Discover & Isolate
- Myocardiac Stem Cells
- Skeletal Muscle Stem Cells
- Adipose Stem Cells
- Neural Crest Stem Cells
- Vascular Adventitial Stem Cells
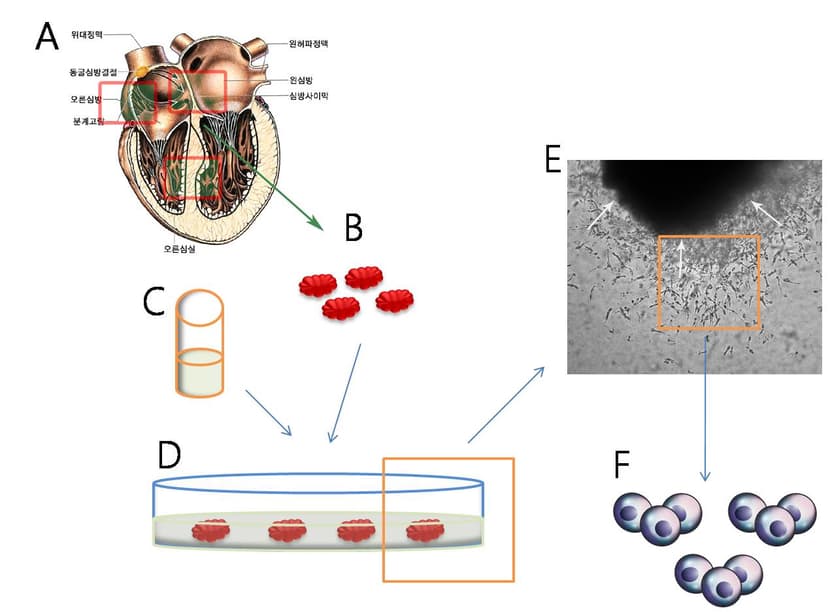
.png&w=828&q=75)
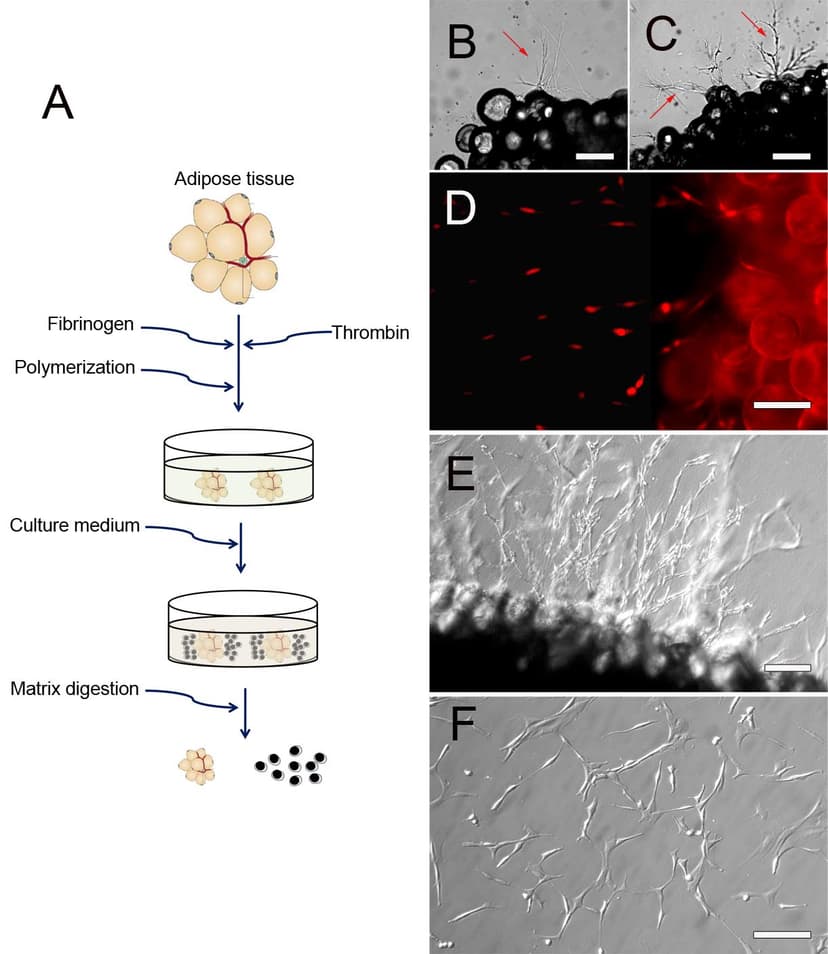
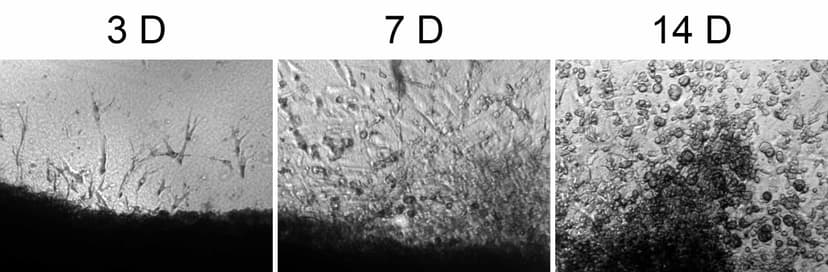
2012
Adapt a Platform Technology to Isolate Synovial Stem Cells
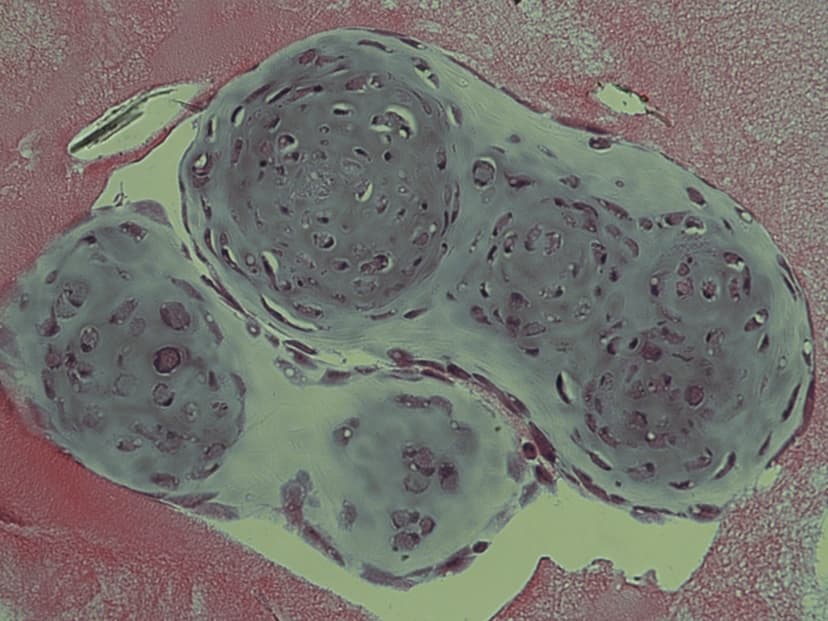
2014
Design a One-Step Integrated Method to Manufacture a Multilayered Cell Sheet of Myocardial Stem Cells
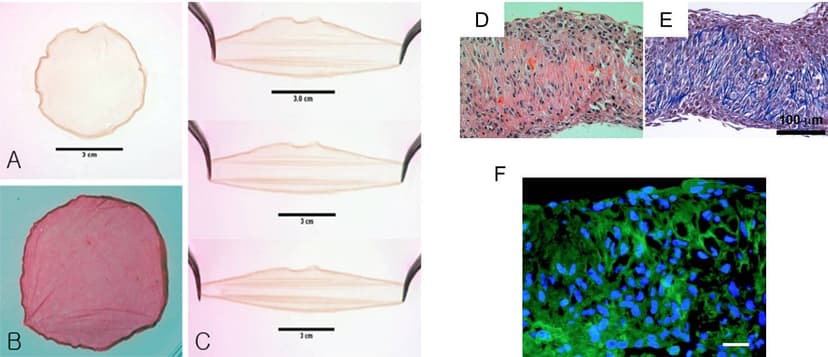
2015
Extend a Cell Sheet Technology to Develop Neural Crest Stem Cells Cell Sheet
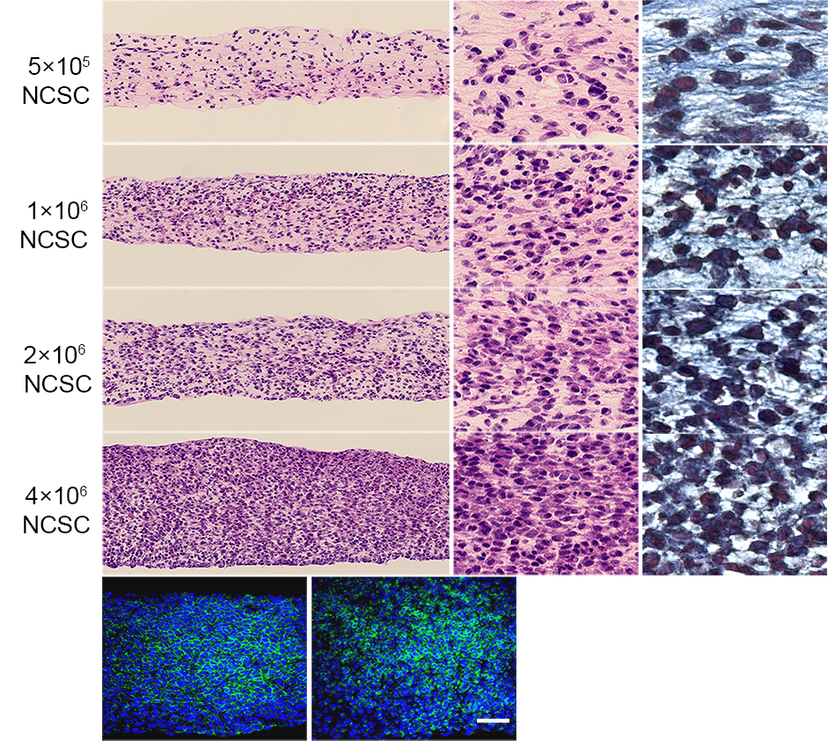
2016
Develop Injectable Microtissue for Wound Healing/Angiogenesis
2017
Expand into Fabricating Neural Microtissue for Neural Tissue Regeneration
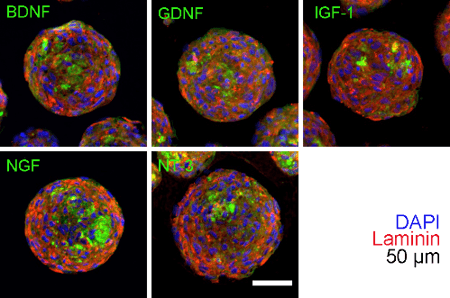
2022
Develop Chemical-define Cryopreservation Formula for Cellular Biodrugs
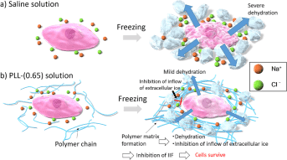
2023
Discover a Platform Technology to Activate and Isolate uPAR+/Nestin+ High Potent Stem Cells
- Myocardium
- Peripheral Nerve
- Skeletal Muscle
- Adipose Tissue
- Synovium
- Bone Marrow MSCs
- Spinal Cord
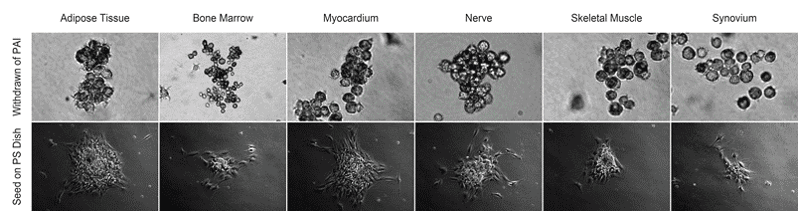
2024
Design a Extracellular Microenvironment for Physical Cell Stretching to Produce Functional EV Production
Newsroom
Stay updated with our latest research, press releases, and corporate announcements.
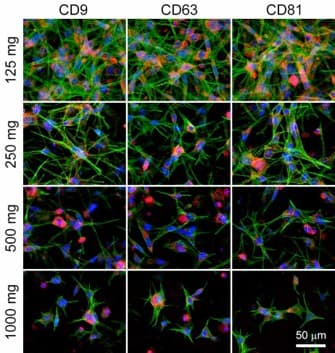
In May 2025, Innostem Bio succeeded in gaining IND approval for a Phase 1 clinical trial of 'ISB-NS02', a therapeutic candidate for traumatic brain injury. The trial aims to regenerate damaged brain tissue and reduce inflammation using adult stem cells derived from peripheral nerves and microtissues. Safety will be assessed in 12 patients, with the trial expected to run until May 2027.

A government-supported clinical study on stem cell treatment for spinal cord injury patients, led by Professor Kim Geung-nyun of Yonsei Severance Hospital, has reached its final stages. The study, targeting acute and subacute spinal cord injury patients, showed improved limb function in patients with incomplete injuries after six months of treatment. The effective verification of this treatment could lead to its application for peripheral nerve diseases or cerebral infarction.
Presented the clinical trial results of ISB-NS01 for spinal cord injury patients under the title 'Potential Role of Adult Peripheral Nerve-Derived Stem Cells in the Central Nervous System' in the Clinical Application of Stem Cells Section.
On August 22, 2024, Innostem Bio participated in the '2024 Exosome Innovation Symposium' at Cha Hospital, where they presented on the development and commercialization potential of exosome therapies and engaged in related networking.

In August 2023, at the Regenerative Medicine Research Achievement Exchange Meeting held in Busan, Innostem Bio presented its achievements in establishing a clinical trial cell bank. Various institutions shared the excellence and future direction of regenerative medicine technologies, including stem cells, organoids, immune modulation, and tissue regeneration.
Presented on 'Myocardium-Resident Stem Cells Exhibiting Both Direct and Indirect Angiogenic Potential', discussing the evidence and mechanism of action for applying myocardium-derived stem cells (ISB-HS01) as a therapeutic for vasculo-regenerative therapy.

Professor Youngil Yang and Professor Jang Jae-sik of Innostem Bio are participating in the 'Development of Cryopreserved Myocardium-Derived Allogeneic Stem Cell Therapy for Patients with Critical Limb Ischemia' project, which is part of the Pan-Ministerial Regenerative Medicine Technology Development Project supported by the Ministry of Science and ICT and the Ministry of Health and Welfare.